Vitamins are essential substances, which ensure the correct functionality of the body. Many different food and food supplements contain vitamins and are available on the market. Especially food supplements enjoy increasing popularity and the regulation as a foodstuff makes ensuring the composition and safety a very important task. The challenging task is to analyze both major groups of vitamins, i.e. water- and fat-soluble vitamins in one analytical run. The used methods are time-consuming. Furthermore, a number of additional substances like sweeteners, acidifiers and antioxidant agents can be found in these preparations. Therefore, a method based on NMR spectroscopy as a fast, reliable, and precise analytical method for the determination of the majority of vitamins as well as accompanying substances without the need for time-consuming sample preparation is developed.
Sample preparation
First the tablets and the capsules were pulverized. Then an appropriate amount of the sample was weighed and solved in a thymol solution (1mg/mL in MeOD). The solution was centrifuged and the extract was removed carefully and placed in a separate flask. Two additional extractions with pure MeOD were made and combined with the first one. The extracts as mixture were transfered into a NMR tube for direct measurements.
Results
As expected, the 1H-NMR spectrum of multivitamin preparations includes a number of signals according to different vitamins, additional substances and the matrix. To find out which signal belongs to which molecule a stock solution has to be prepared for each active ingredient. Then 50 – 100 µg of each stock solution is put alternating into the sample solution and a new 1H-NMR spectrum was recorded. The signals belonging to the active ingredient increase, so you have find out the NMR integration regions shown in Tab. 1.
Tab. 1 NMR integration regions used for quantification
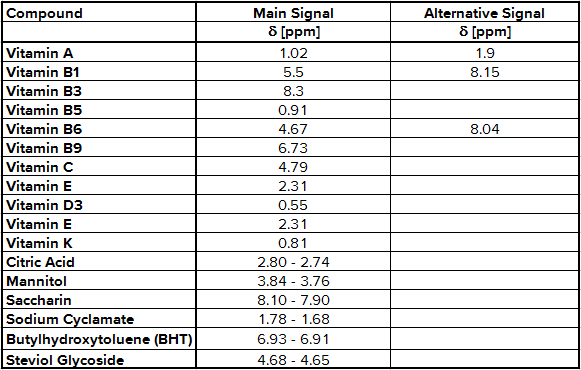
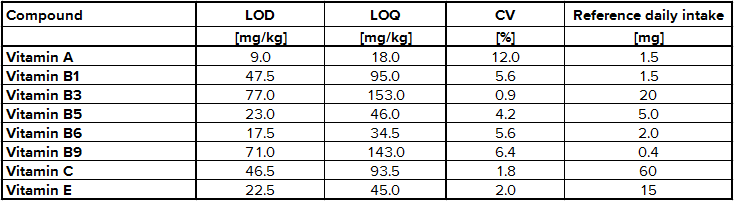
The determination of content of the different substances by quantitative NMR needs an internal standard, in this case thymol. The internal standard and sample were weighed exactly. With the signal intensity, the number of atoms and the weight it is possible to calculate the content of each known substance. Detailed NMR spectra of an exemplary sample is shown in Fig. 1. Vitamin B2 was not detected due to its low solubility in methanol; vitamin B7 was not quantified due to spectral overlap; vitamin B12, D3 and K were not detected by NMR (below LOD). The LODs were found between 9.0 mg/kg for vitamin A and 77.0 mg/kg for vitamin B3. LOQ values varied between 18.0 mg/kg and 153 mg/kg (Tab. 2). Ascorbic acid is a special case because of its stability. There is an equilibrium between ascorbic acid and its first oxidation product, dehydroascorbic acid (DHAA). Over time the signal intensity of ascorbic acid decreases. A lower signal intensity means that the quantification becomes incorrect and you determine a lower content of ascorbic acid. Therefore, the measurement should be made a short time after extraction to minimize the error. To stabilize ascorbic acid and to avoid oxidation often citric acid is part of multivitamin preparations. This method is a suitable tool for simultaneous determination of vitamins and other additional substances in multivitamin preparations.
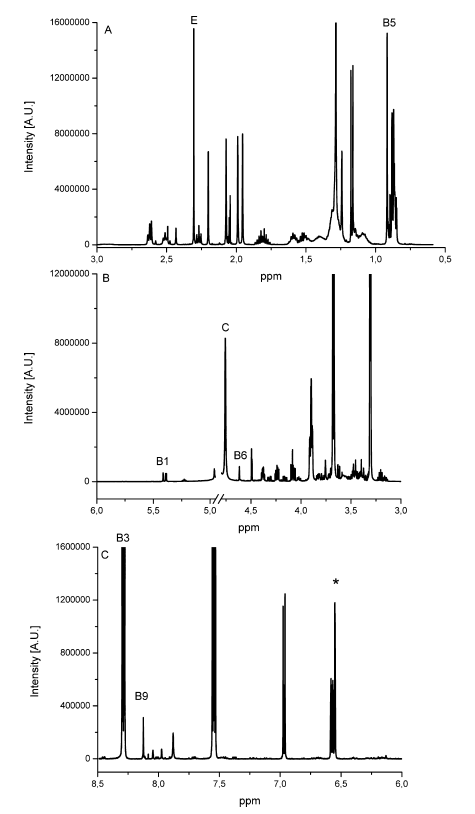
Fig. 1 Detailed NMR spectra of an exemplary multivitamin preparation. The peaks of each individual vitamin and internal standard (*) used for quantification are depicted on the spectrum [1]
Tab. 2 Validation results for selected vitamins using NMR
The research was performed by Spectral Service AG and fully adopted by Steelyard Analytics Inc.


Recent Comments